
One of the development trends in the chemical industry is that the safe, clean, efficient, energy saving and sustainable. Continuous flow chemistry is one of the important technologies that LinkChem supports in green chemistry. Based on industrial continuous flow reaction technology and adhering to the concept of green and safe production, it provides customers with continuous flow route design, process route optimization, laboratory testing, catalyst development, and factory feasibility studies, continuous flow equipment improvement, process development and overall equipment packaging solutions and other services to better meet customer needs for large-scale, sustainable production.

1.Process development capability
LinkChem's flow chemistry team has rich experience in process development and optimization, and has an in-depth understanding of chemical reactions and the working principles of microchannel reactors. Through perpetual innovative development and design, it can implement multi-step continuous processes for multiple products and improve society effect and economic effect, and achieve safe, green and sustainable production from laboratory to industrialization for customers.
2.Catalyst development capability
LinkChem focuses on exploring new green synthesis methods, developing new catalyst carrier materials, and deploying a catalyst team led by PhDs. It has achieved the combination of new catalyst technology and continuous flow technology on multiple projects, achieving accelerated reaction speeds. , improve productivity, save energy and raw materials, and provide new solutions for green chemical reactions.



3.Engineering design capability
LinkChem relies on the engineering and technical team's professional knowledge in the fields of commercial engineering management, factory equipment management and chemical process simulation, as well as many years of experience in design, to carry out engineering design according to corresponding requirements for different reaction processes:
| Our Discovery Services covers the entire spectrum of early-stage research from target identification to delivery of drug candidates for further development. We have experienced team of medicinal chemistry and organic chemistry experts to support our customers’ need for hit identification, lead generation lead optimization programs. | |
|
 |
|
As an experienced commercial manufacturer, LinkChem can provide one-stop outsourcing intermediates services of process route development and verification, analysis method development and verification, process improvement and application registration to help customers with investigational to commercial-scales developments. The development services include a large number of raw materials, registered starting materials and GMP advanced intermediates throughout the entire drug development process. With our strong and diverse infrastructure, LinkChem facilities are multipurpose and flexible and allow a rapid response to meet customer requirements. LinkChem have the advantage in the field of Heterocyclic Chemistry, chiral drugs, Carbohydrate chemistry, Nucleoside chemistry, Peptide Chemistry and Antibody Drug Conjugation (ADC). |
 |

|
LinkChem’s factory workshop has good versatility, flexible capacity of rapid switch and project management mode to ensure that the production and operation system can quickly and high-quality delivery of products and services required by customers. Relying on the international standard quality system and large-scale commercialized GMP product production experience, we can provide customers with high quality commercialized API and GMP intermediate products at the optimal cost, and provide customers with a series of additional services such as secondary process development and optimization. LinkChem has different types and different sizes of plants, which can achieve the perfect combination of laboratory scale to commercial production scale and ensure the stability of supply. |
 |
|
|
|
|
|
Service Platform: XFlow Chemistry is committed to building the flow chemistry platform using the industrial continuous flow reaction technology, adhering to the concept of green and safe production, providing customers with continuous flow process design, process optimization, laboratory testing, feasibility study, equipment improvement, process development as well as fully integrated solutions to better meet the needs of customers in large-scale, sustainable production. |
 |
|
|
|
|
| LinkChem has a highly experienced regulatory affairs team which provides CMC services at all research stages of Investigational New Drug application (IND) and its clinical trial phases (P1, P2, P3), New Drug Applications (NDA), Abbreviated New Drug Application (ANDA), and post-approval regulatory services including product change, annual report and re-registration service. LinkChem establishes standardized management system to ensure the authenticity and professionalism of the data, to assist the drug regulatory department verification. With profound professional knowledge in synthesis, QC, QA, domestic and foreign policies and regulations, registration and rich industry resources, we provide domestic and foreign customers with high efficiency and high quality drug registration and declaration services in accordance with the requirements of laws and regulations. |
 |
|
LinkChem adheres to the strategy for synchronous development in fields of pharmaceutical, new energy and advanced materials. We make the brand of LinkChem with leading service in pharmaceutical CDMO industry, and exploit the field of advanced materials relying on existing pharmaceutical business mode.
Based on rich experience in technology development and accumulation, we can provide one-stop solutions about technology R&D, key process optimization, end-product quality control and commercial manufacturing for advanced materials (photoresist monomer, PI monomer, PSPI, lithium battery additive, functional monomer, etc.) since 2015. Until now, a number of products have passed sample test, and some have been in abundant supply.



CCL Resin monomer,Photoresist monomer,PI monomer,PSPI,Lithium battery additive, etc.
| No. | Category | Product | CAS No. | Structural Formula |
|---|---|---|---|---|
| 1 | CCL Resin Monomer | BVPE | [48174-52-3] |
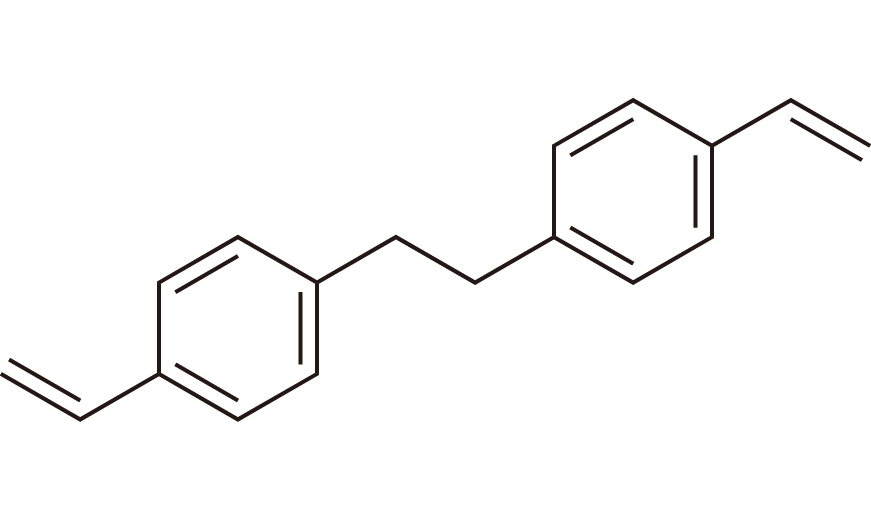
|
| 2 | pBVF | [2842071-43-4] |
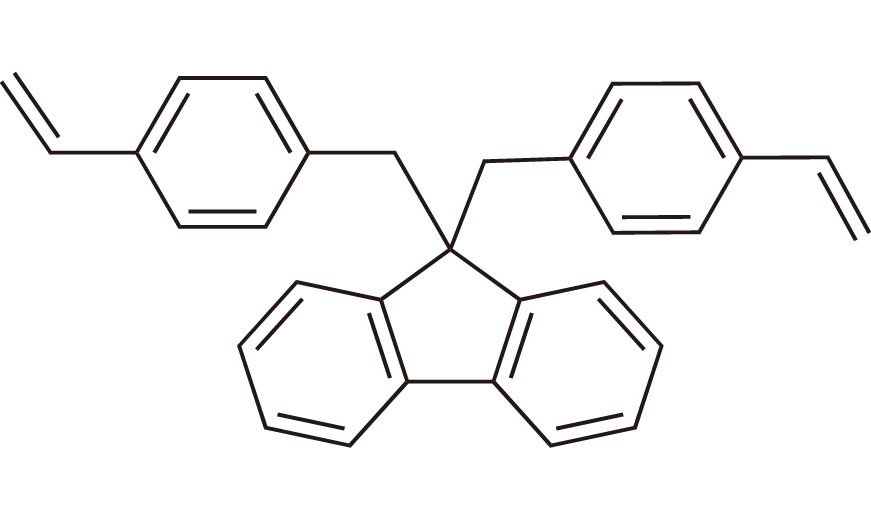
|
|
| 3 | BCB | [694-87-1] |
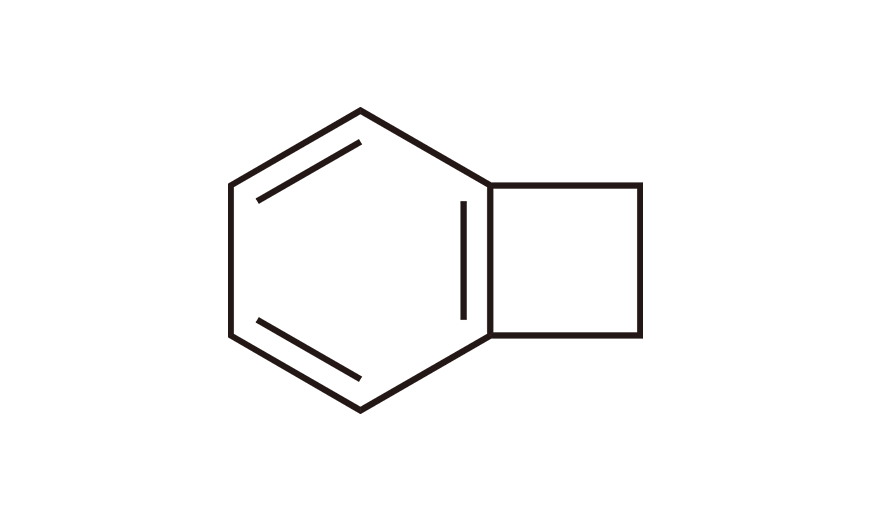
|
|
| 4 | BrBCB | [1073-39-8] |
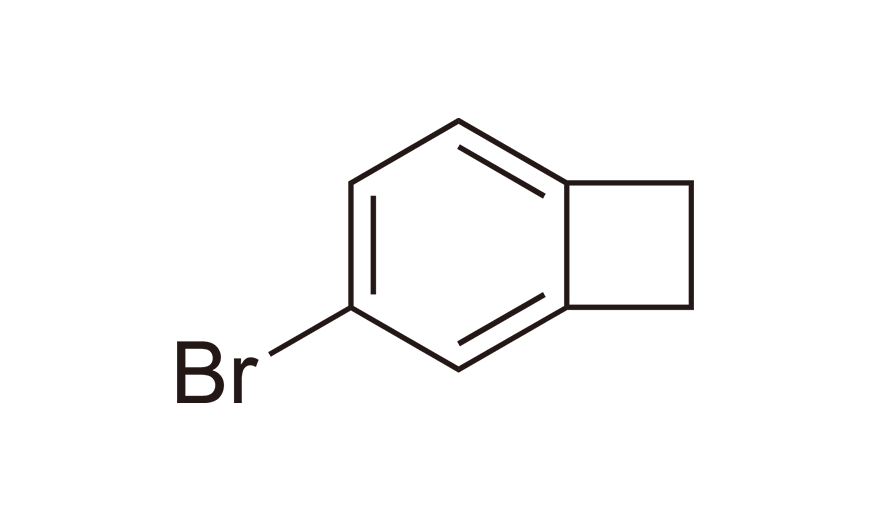
|
|
| 5 | VBCB | [99717-87-0] |
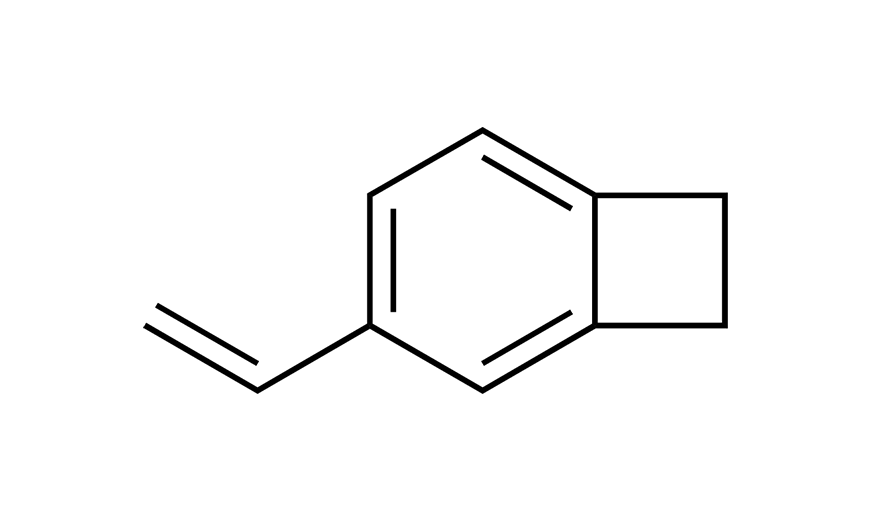
|
|
| 6 | PI Monomer | MCTC | [73003-90-4] |
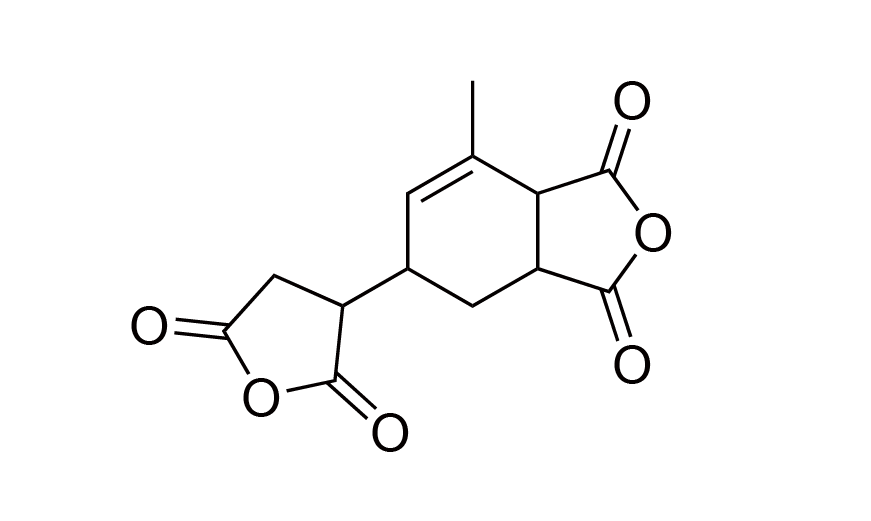
|
| 7 | CBDA | [4415-87-6] |
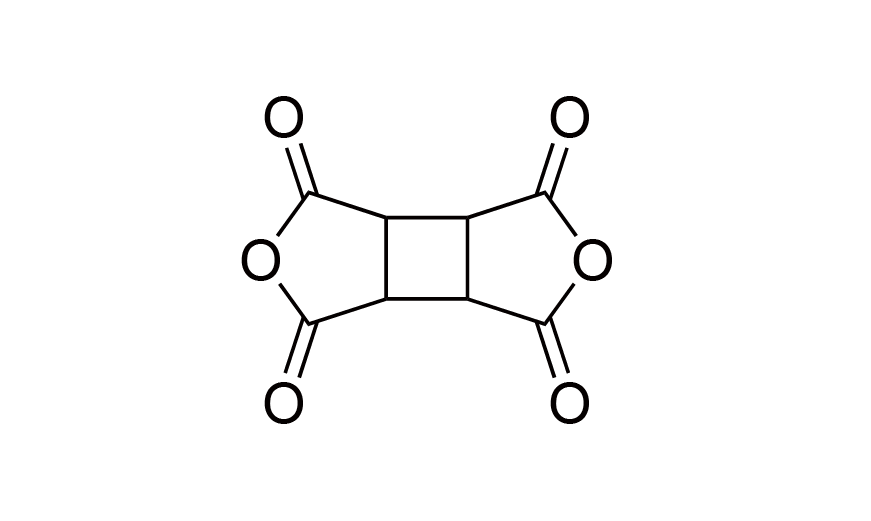
|
|
| 8 | DMCBDA | [137820-87-2] |
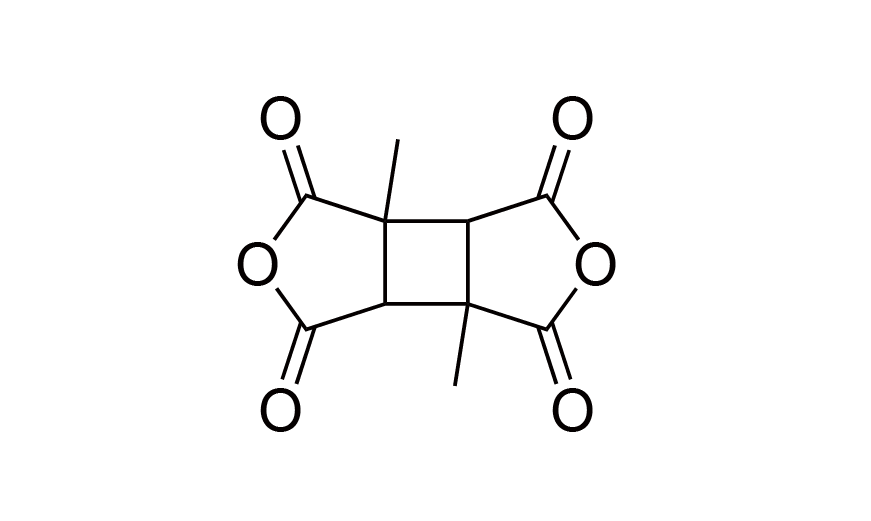
|
|
| 9 | ODA | [101-80-4] |
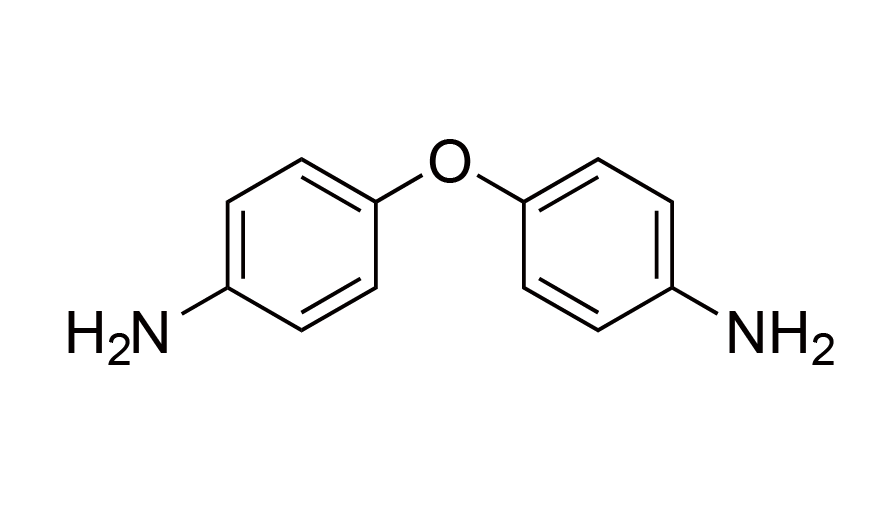
|
|
| 10 | mTD | [84-67-3] |

|
|
| 11 | BAPB | [13080-85-8] |
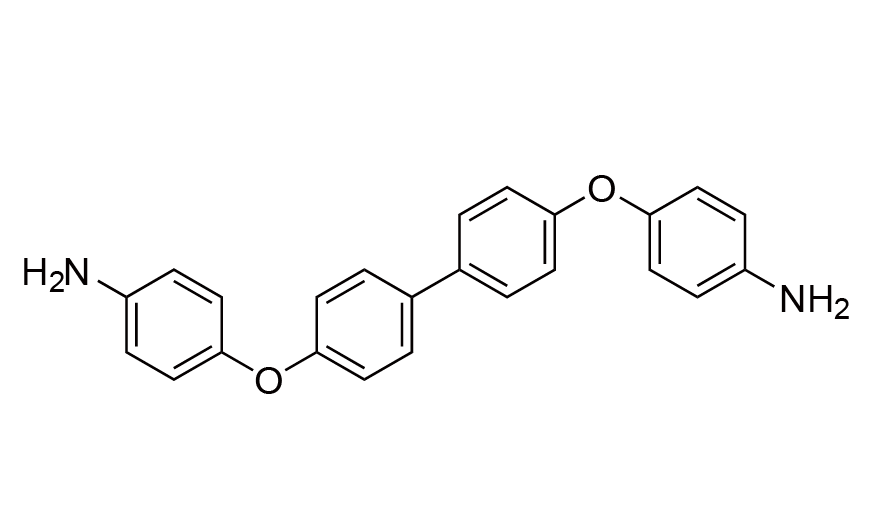
|
|
| 12 | CFDA | [107934-68-9] |
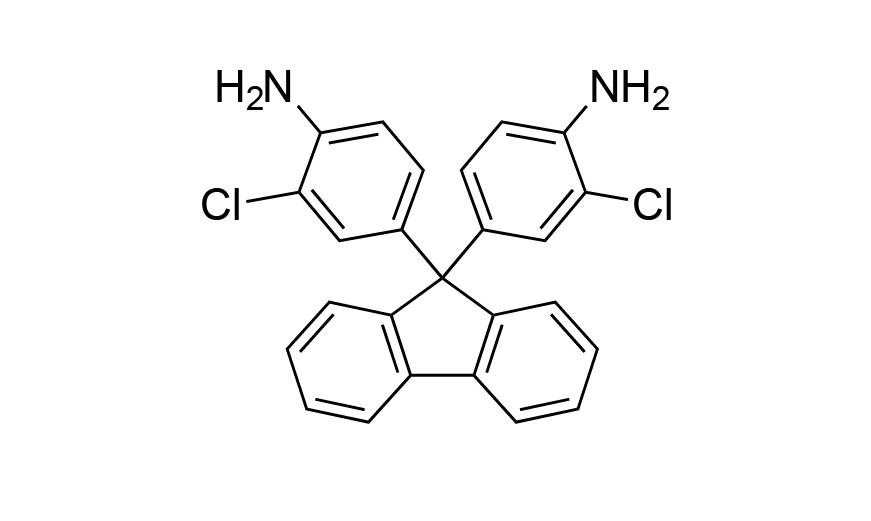
|
|
| 13 | FDA | [15499-84-0] |
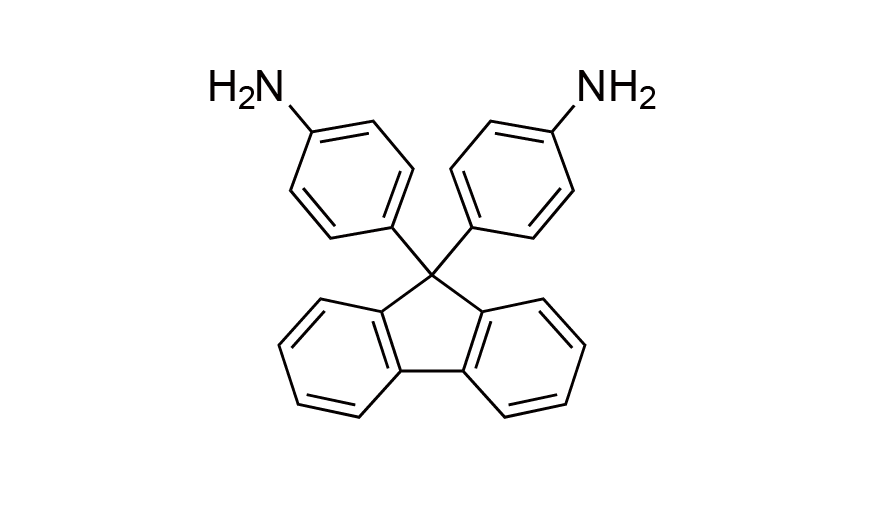
|
|
| 14 | N/A | [2716984-43-7] |
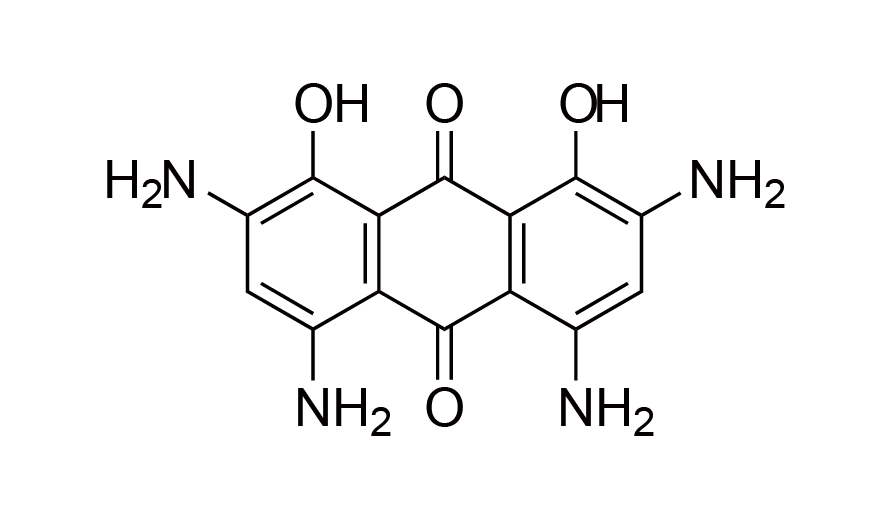
|
|
| 15 | N/A | [3102-70-3] |
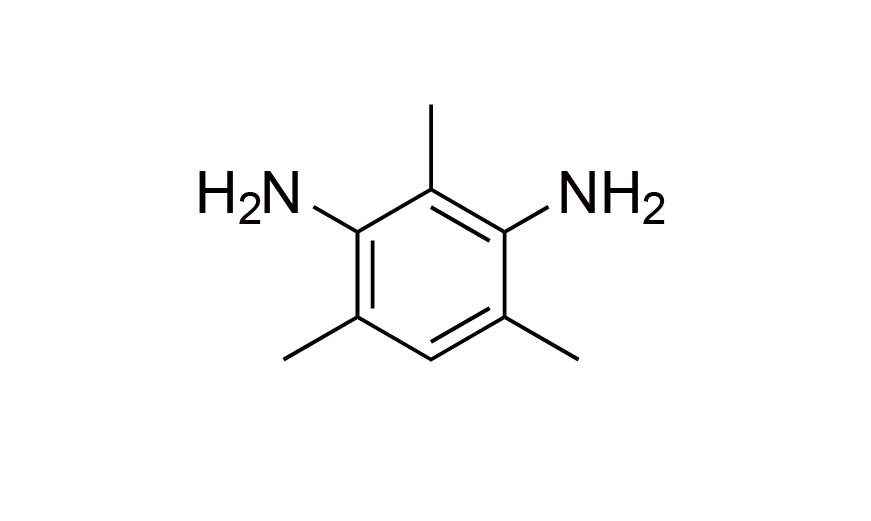
|
|
| 16 | PSPI Monomer | BAP | [1220-78-6] |
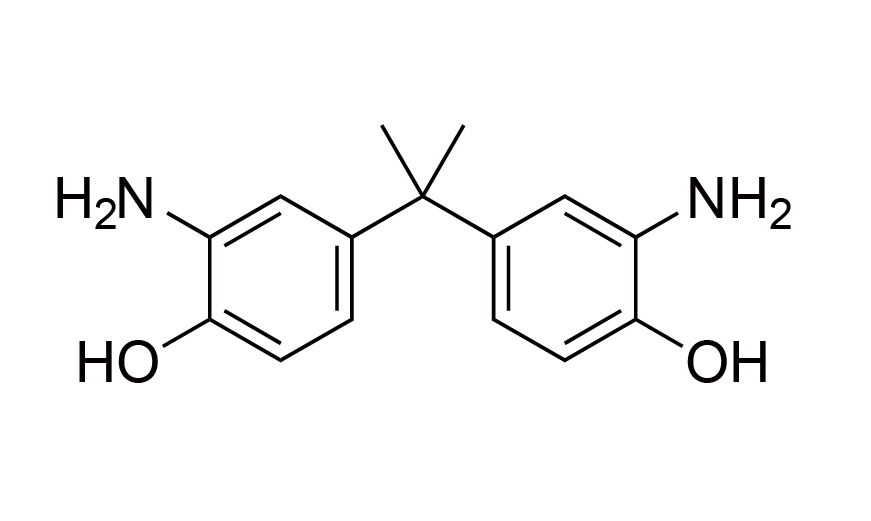
|
| 17 | OBAP | [6423-17-2] |
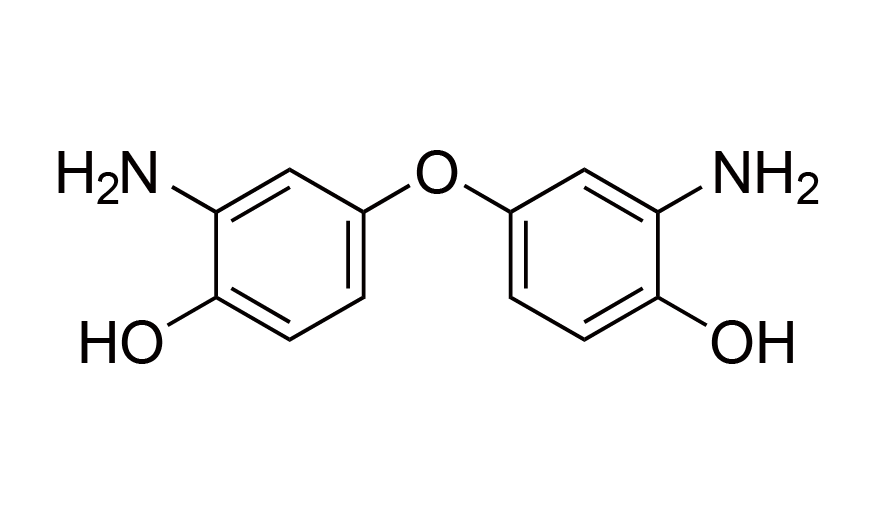
|
|
| 18 | CHPS | [30817-90-4] |
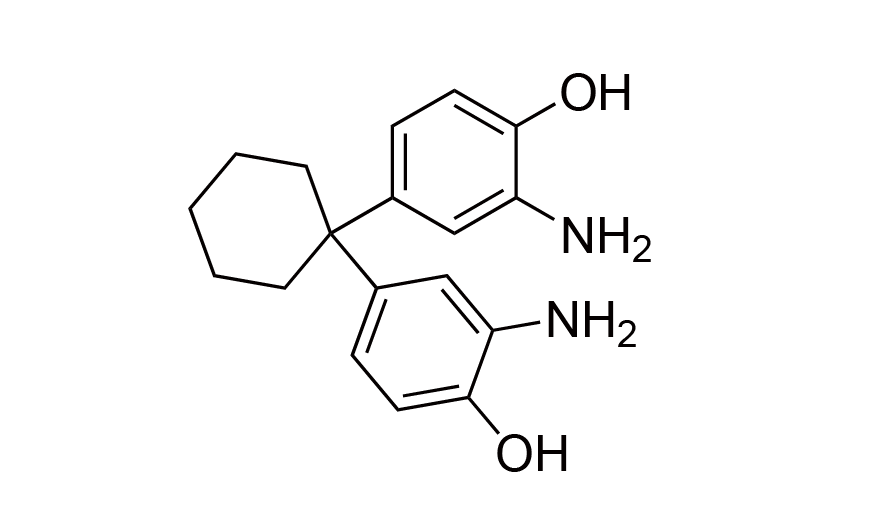
|
|
| 19 | BAHS | [7545-50-8] |
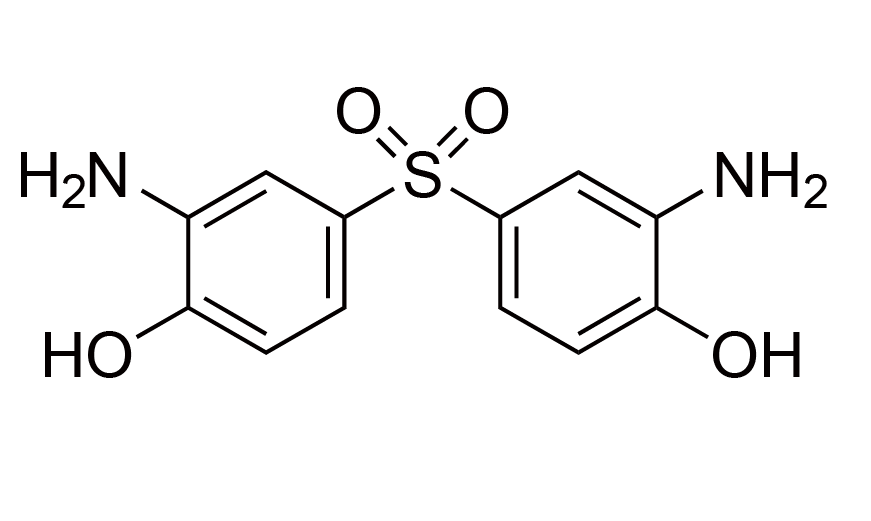
|
|
| 20 | Photoresist Monomer | VPBO | [95418-58-9] |
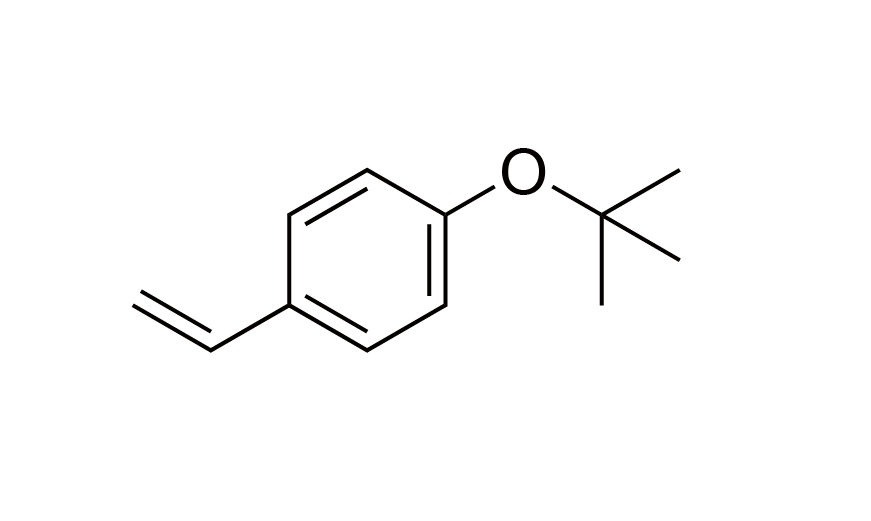
|
| 21 | Lithium Battery Additive | VC | [872-36-6] |
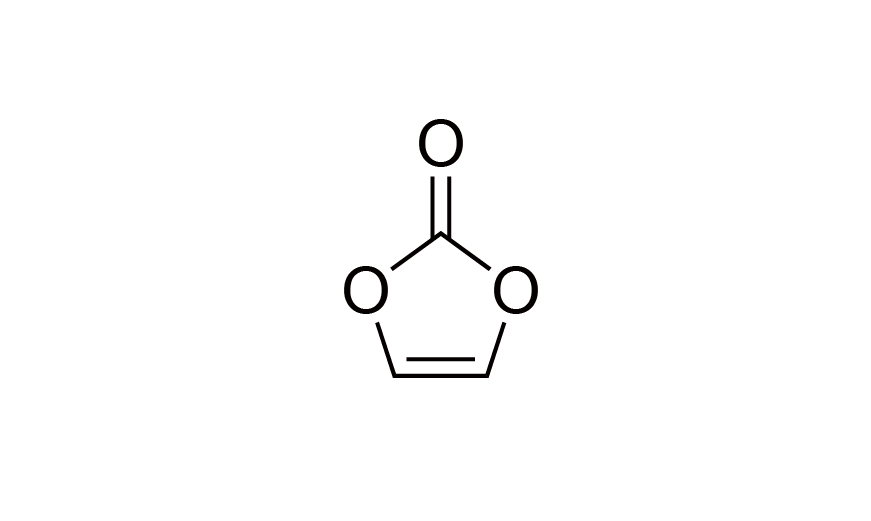
|
| 22 | LiFSI | [171611-11-3] |

|
|
| 23 | Functional Monomer | CHDA | [1076-97-7] |
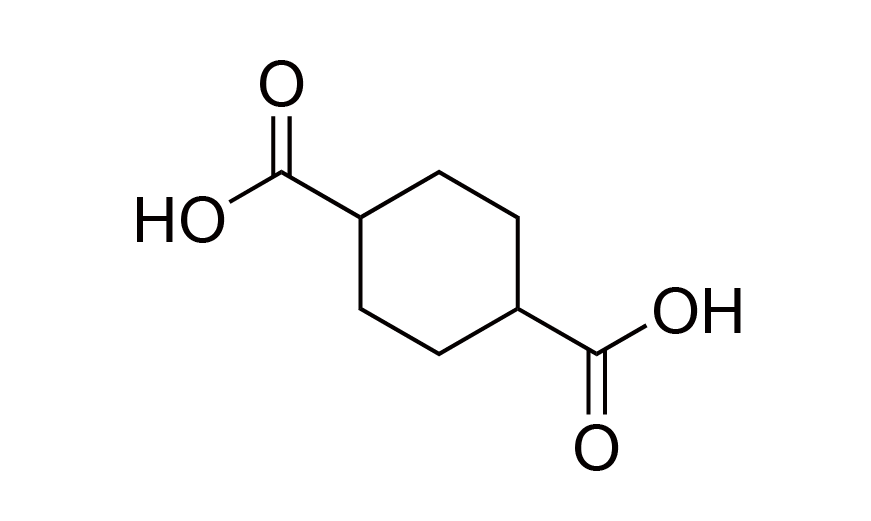
|
| 24 | N/A | [97344-49-5] |
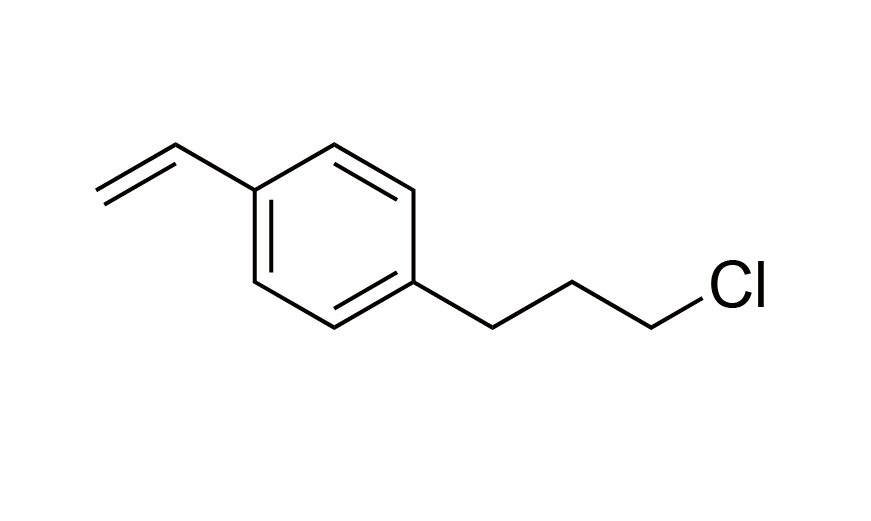
|
|
| 25 | N/A | [1204-10-0] |
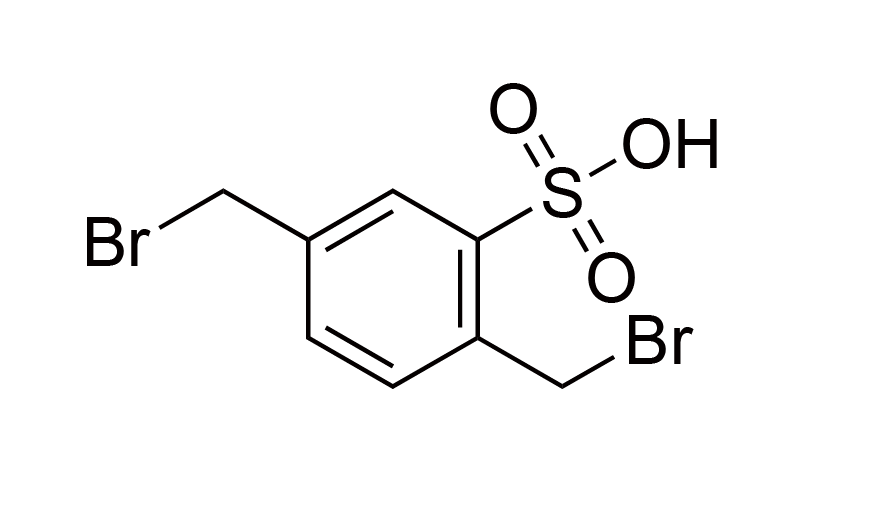
|
|
| 26 | N/A | [128481-73-2] |
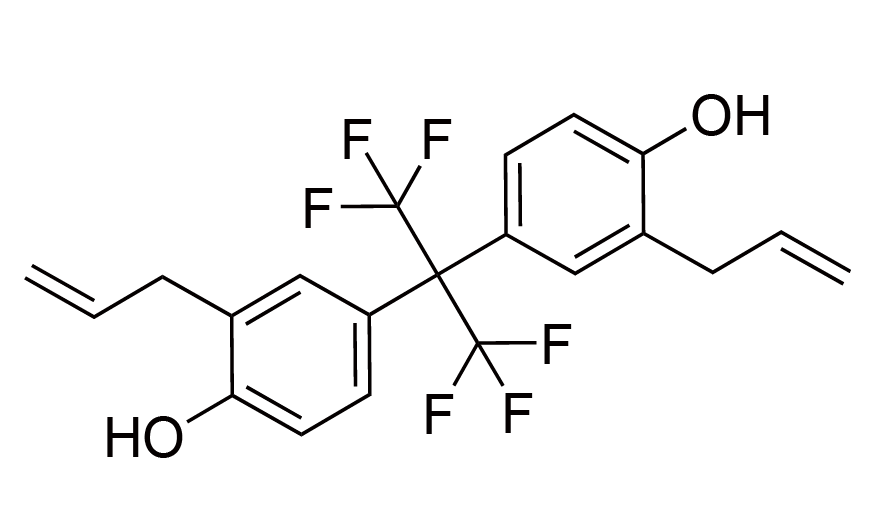
|
|
 |